Emerging markets are becoming progressively important to the growth of clinical trials, which is causing revolution in the worldwide clinical research environment. The industry, which has historically been controlled by North America and Western Europe, is progressively moving towards regions that have contact to diverse populations, faster patient recruitment, and cost capabilities. The demand for good quality clinical data, the development of therapeutics, and the growing global occurrence of ailments are the core drivers of this development.
For CROs and therapeutic businesses, emerging markets such as Asia-Pacific, Latin America, Eastern Europe, and parts of the Middle East and Africa provide noteworthy prospects. These areas provide contact to large inhabitants of newly diagnosed patients, lesser operational charges, and improved healthcare set-up.
Key Drivers of Growth in Emerging Markets
Emerging markets are now the main hubs for all the therapeutic researches, clinical trials and pharmaceutical business. Following are the main factors that has high input in the growth of trials.
Rising Number of Patients: A large group of untreated patients permits for faster enrollment and diverse data congregation.
Fewer Competing trials: Result in a faster recruitment process, ultimately reducing trial timelines.
Lower Costs: Budget-friendly site work, doctor fees, and patient care costs reduce the total price of trials.
Established Regulatory Framework: Governments are making approval steps simpler shortening wait times, and lining up with global standards.
Better Resources: Money put into health facilities digital health tech, and trained research teams boosts the ability to run trials.
Emerging Markets and Comparator Trials
Pharmaceutical companies are progressively using comparator trials. These clinical trials evaluate how effective a new drug is compared to previously approved treatment instead of just a placebo. This comparator approach helps companies show that their medicine is both safer and effective, which can advance the probability of getting FDA approval. Consequently, many researchers look to emerging markets in Asia and Latin America, where the cost of comparator drugs is typically lesser. Nevertheless, import principles and shipping cost a lot. Luckily, emerging markets are working to improve their regulations, targeting for clearer guidelines and improved drug safety. Countries like Pakistan, India, and China have set up activities to achieve drug sourcing and protect confidential documents.
Leading Emerging Markets in Clinical Research
Emerging markets are reshaping the clinical study environment, with a number of regions becoming significant players due to their developing pharmaceutical industries, improved regulations, and rising healthcare spending.
In the Asia-Pacific region, countries including South Korea, China, India, and Pakistan are leading rapid pharmaceutical expansion. Government is getting advanced in healthcare about regulatory submissions and investments in clinical trials and because of these regulations global sponsors finding these countries as a core hub.
Eastern Europe, which covers Poland and Hungary, is well-recognized for its expert team in clinical studies, regulatory approvals and faster patient recruitments.
Latin America particularly Brazil, Mexico, and Argentina are becoming a noteworthy center for clinical study due to governing enhancements.
Finally, the healthcare revolution in the Middle East and Africa is having a substantial constructive impact on nations like South Africa, Egypt, and the United Arab Emirates.
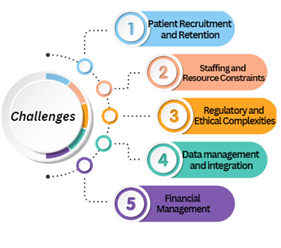
Challenges in Conducting Clinical Trials in Emerging Markets
Strategies for Success in Emerging Markets
In emerging market trends, success of clinical trial depends on a strategic approach. For integration of strategies a good policymaking process, technological advancements and regulatory submissions are very important for optimizing trial outcomes. Having strong relationships with CROs and regulatory experts supports advancements and clinical trial success rates. Likewise, staying agile and adjusting according to market needs as decentralized clinical trials and patient centric approaches results in evolving clinical research environment.

Future Outlook and Opportunities in Emerging Markets
Emerging markets continue to invest in regulatory developments, infrastructure, technology and are likely to play a key role in global clinical trials in the future. The future of clinical trials in emerging markets is promising as increasing agreements between global sponsors and local research management organizations drive innovation and improve study effectiveness.
Pharma companies and CROs are actively networking with healthcare organizations to streamline clinical trials. These partnerships help bridge the gap between global expertise and local intuition, fostering a more sustainable research environment.
Additionally, emerging trends such as decentralized trials, digital health innovations, and AI driven data analytics are transforming clinical trials in these regions.
Conclusion
At Orci Trials, we recognize the potential of emerging markets in shaping the future of Clinical research. These regions are ideally suited to conduct high-quality, high impact trials due to their cost-effectiveness, diverse patient populations, and short recruitment periods.
As a leading CRO, we strive to leverage these opportunities to revolutionize clinical research. With a focus on quality and ingenuity, Orci Trials is committed to transforming clinical research in emerging markets and ultimately improving patient outcomes around the world.